11+ pages in a blast furnace iron iii 1.8mb. To make steel iron ore is first mined from the ground. Fe2O3 Get the answers you need now. Carbon seems to play a significant role in influencing the brittleness and hardness balance in iron. Read also iron and understand more manual guide in in a blast furnace iron iii In a blast furnace fuel ores and flux are continuously supplied through the top of the furnace while a hot blast of air is blown into the lower section of the furnace.
Iron is made by reacting iron ore iron oxide and impurities coke a reductant and limestone CaCO 3 in a blast furnace. Generally the extraction of metals and their isolation are based on three major procedures.

Iron Extraction W3spoint
| Title: Iron Extraction W3spoint |
| Format: ePub Book |
| Number of Pages: 213 pages In A Blast Furnace Iron Iii |
| Publication Date: February 2020 |
| File Size: 5mb |
| Read Iron Extraction W3spoint |
 |
A The balanced chemical reaction is FeOs 3COg.
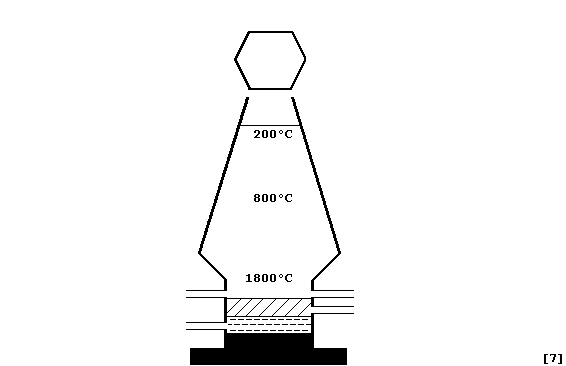
Iron ores such as haematite contain iron III oxide Fe2O3. In this reduction reaction oxygen is removed from the iron III oxide to leave behind iron. Blast furnace Wikipedia Overview. This is called Pig Iron. A blast furnace is a type of metallurgical furnace used for smelting to produce industrial metals generally pig iron but also others such as lead or copper. Fe2O3s COg Fes CO2g a If 500 kg Fe2O3 are available to react how many moles of CO are needed.
How Can Iron Be Separated From The Oxygen In Its Ore Quora
| Title: How Can Iron Be Separated From The Oxygen In Its Ore Quora |
| Format: PDF |
| Number of Pages: 190 pages In A Blast Furnace Iron Iii |
| Publication Date: September 2018 |
| File Size: 1.4mb |
| Read How Can Iron Be Separated From The Oxygen In Its Ore Quora |
 |
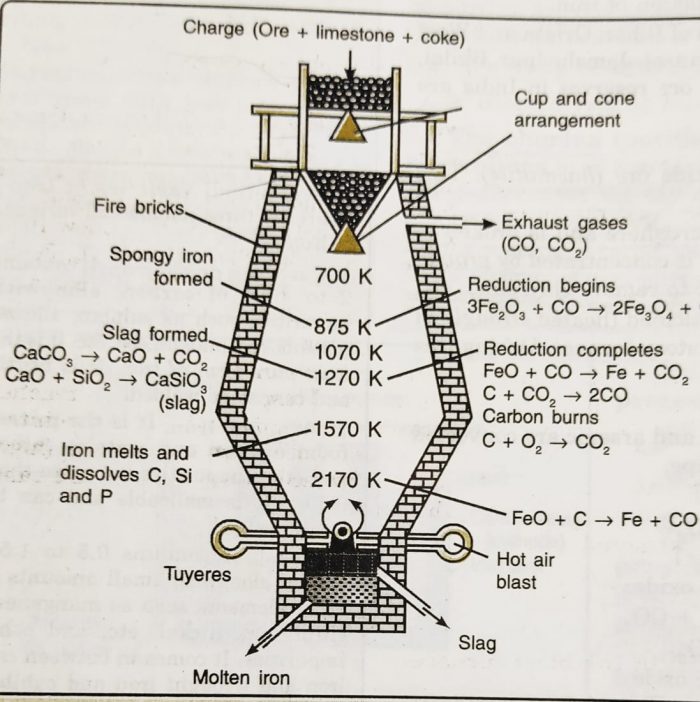
Extraction Of Iron Class 12 General Principles And Processes Of Isolation Of Elements
| Title: Extraction Of Iron Class 12 General Principles And Processes Of Isolation Of Elements |
| Format: ePub Book |
| Number of Pages: 166 pages In A Blast Furnace Iron Iii |
| Publication Date: October 2021 |
| File Size: 1.35mb |
| Read Extraction Of Iron Class 12 General Principles And Processes Of Isolation Of Elements |
 |
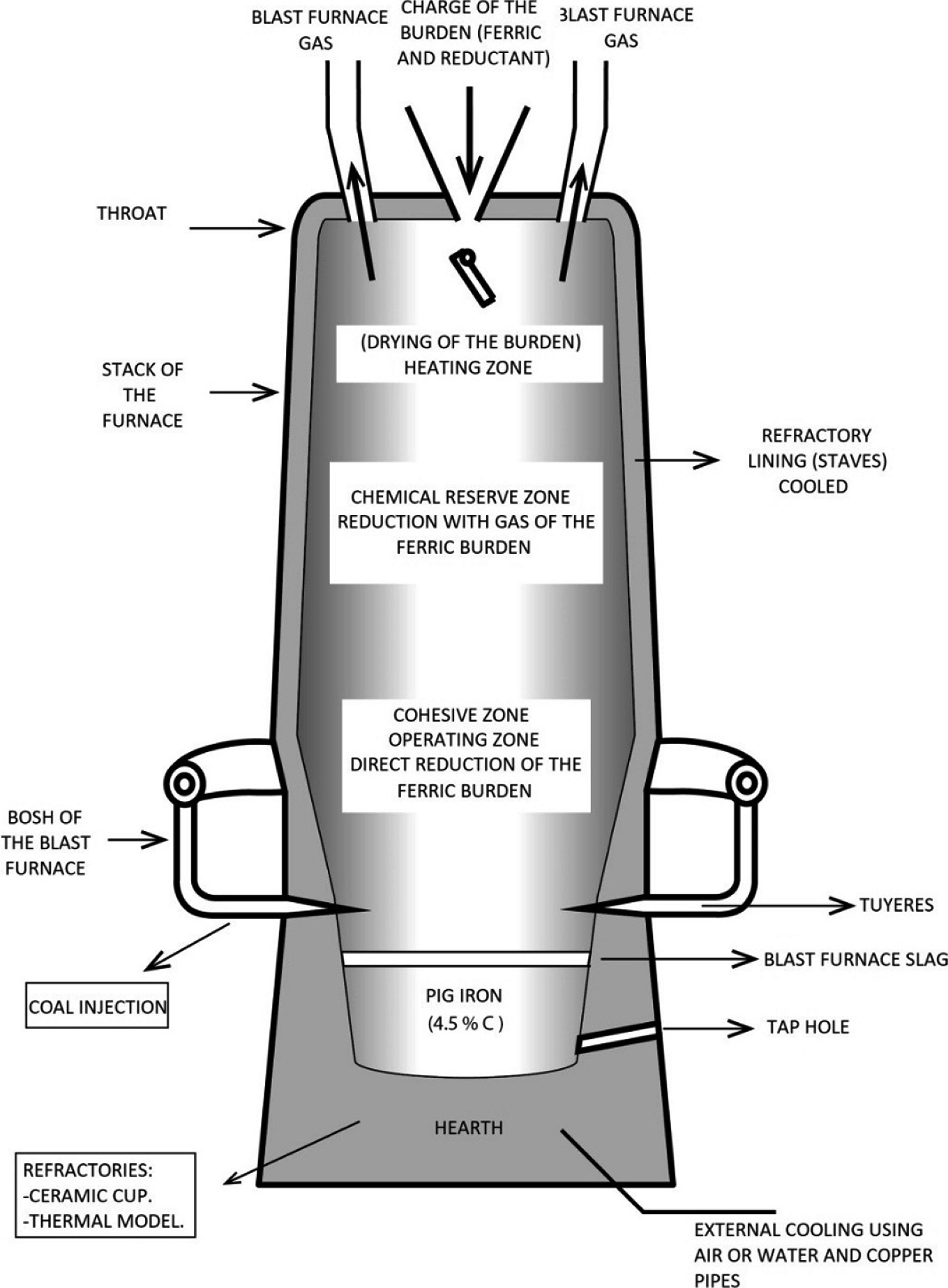
Production Of Iron In The Blast Furnace Springerlink
| Title: Production Of Iron In The Blast Furnace Springerlink |
| Format: PDF |
| Number of Pages: 172 pages In A Blast Furnace Iron Iii |
| Publication Date: December 2021 |
| File Size: 2.3mb |
| Read Production Of Iron In The Blast Furnace Springerlink |
 |
Lesson Explainer Extracting Iron Nagwa
| Title: Lesson Explainer Extracting Iron Nagwa |
| Format: PDF |
| Number of Pages: 314 pages In A Blast Furnace Iron Iii |
| Publication Date: June 2019 |
| File Size: 2.3mb |
| Read Lesson Explainer Extracting Iron Nagwa |
 |
Lesson Explainer Extracting Iron Nagwa
| Title: Lesson Explainer Extracting Iron Nagwa |
| Format: ePub Book |
| Number of Pages: 312 pages In A Blast Furnace Iron Iii |
| Publication Date: October 2017 |
| File Size: 6mb |
| Read Lesson Explainer Extracting Iron Nagwa |
 |

Gcse 2 Blast Furnace Extraction Of Iron Recycling Steel Making Reduction Of Haematite Ore Magite Description Of Processs Igcse O Level Ks4 Science Chemistry Revision Notes Revising
| Title: Gcse 2 Blast Furnace Extraction Of Iron Recycling Steel Making Reduction Of Haematite Ore Magite Description Of Processs Igcse O Level Ks4 Science Chemistry Revision Notes Revising |
| Format: eBook |
| Number of Pages: 139 pages In A Blast Furnace Iron Iii |
| Publication Date: February 2019 |
| File Size: 725kb |
| Read Gcse 2 Blast Furnace Extraction Of Iron Recycling Steel Making Reduction Of Haematite Ore Magite Description Of Processs Igcse O Level Ks4 Science Chemistry Revision Notes Revising |
 |

Blast Furnace Iron Extraction Diagram Quizlet
| Title: Blast Furnace Iron Extraction Diagram Quizlet |
| Format: PDF |
| Number of Pages: 342 pages In A Blast Furnace Iron Iii |
| Publication Date: September 2019 |
| File Size: 1.8mb |
| Read Blast Furnace Iron Extraction Diagram Quizlet |
 |
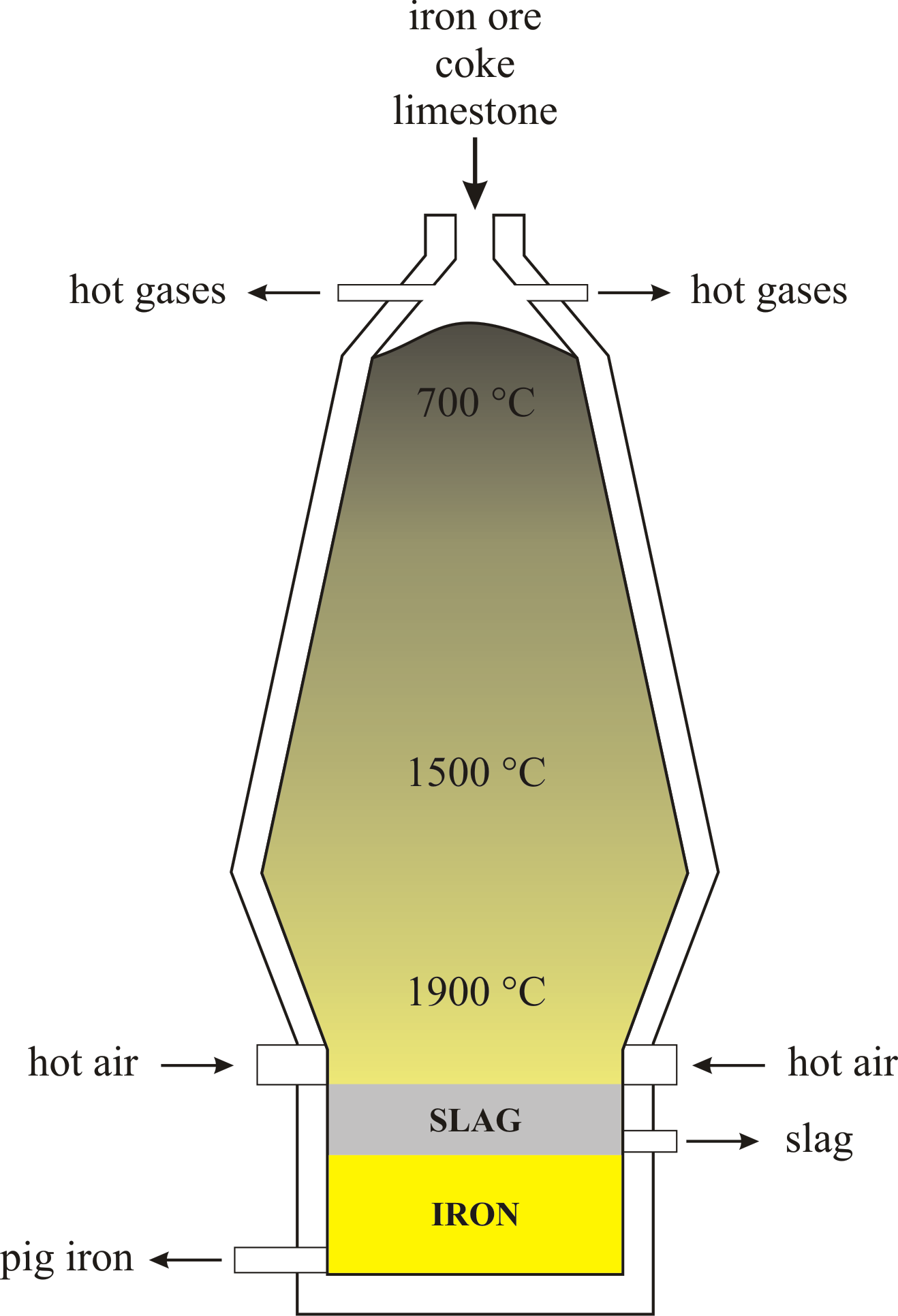
Blast Furnace Chemistry Dictionary Glossary
| Title: Blast Furnace Chemistry Dictionary Glossary |
| Format: eBook |
| Number of Pages: 158 pages In A Blast Furnace Iron Iii |
| Publication Date: January 2020 |
| File Size: 3.4mb |
| Read Blast Furnace Chemistry Dictionary Glossary |
 |

Savvy Chemist Transition Metals Some Chemistry Of Iron 1
| Title: Savvy Chemist Transition Metals Some Chemistry Of Iron 1 |
| Format: ePub Book |
| Number of Pages: 147 pages In A Blast Furnace Iron Iii |
| Publication Date: September 2020 |
| File Size: 5mb |
| Read Savvy Chemist Transition Metals Some Chemistry Of Iron 1 |
 |
Lesson Explainer Extracting Iron Nagwa
| Title: Lesson Explainer Extracting Iron Nagwa |
| Format: ePub Book |
| Number of Pages: 186 pages In A Blast Furnace Iron Iii |
| Publication Date: October 2020 |
| File Size: 1.2mb |
| Read Lesson Explainer Extracting Iron Nagwa |
 |

Aufbau1 Metals Extraction Of Iron
| Title: Aufbau1 Metals Extraction Of Iron |
| Format: ePub Book |
| Number of Pages: 330 pages In A Blast Furnace Iron Iii |
| Publication Date: January 2017 |
| File Size: 2.1mb |
| Read Aufbau1 Metals Extraction Of Iron |
 |
This is called Pig Iron. In a huge container called a blast furnace Iron ores such as haematite contain ironIII oxide Fe 2 O 3 The oxygen must be removed from the ironIII oxide in order to leave the iron behind. Mol b How many moles of each product are formed.
Here is all you need to learn about in a blast furnace iron iii The oxygen must be removed from the iron III oxide in. Iron when extracted from iron ore such as haematite containing iron III oxide Fe2O3 in a blast furnace is called iron extraction blast furnace metallurgy. Haematite is added to the top of the furnace along with coke ie. Aufbau1 metals extraction of iron lesson explainer extracting iron nagwa how can iron be separated from the oxygen in its ore quora production of iron in the blast furnace springerlink savvy chemist transition metals some chemistry of iron 1 lesson explainer extracting iron nagwa In a blast furnace IronIII oxide is used to produce iron by the following unbalanced reactionFe2O3s COg--Fes CO2g if 400 kg Fe2O3 are available to react how many moles of CO are needed.


